Research Interests
Using cutting edge techniques such as mass cytometry (CyTOF) and single cell RNA sequencing (scRNAseq), my group is currently focused on studying how mucosal immunity develops and what goes awry to cause disease.
Immunobiology at the maternal-fetal interfaceUsing a combination of primary cells, organoids and primary tissue, we are investigating how immune dysregulation at the feto-maternal interface can contribute to preterm labor and other pregnancy complications.
|
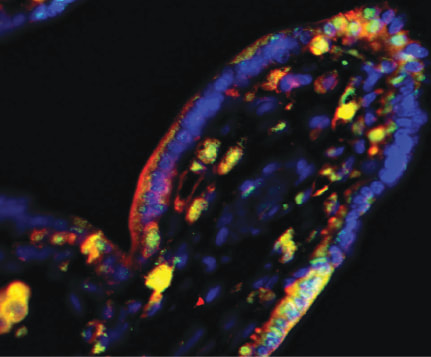
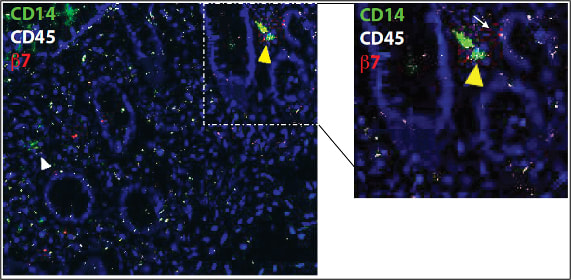
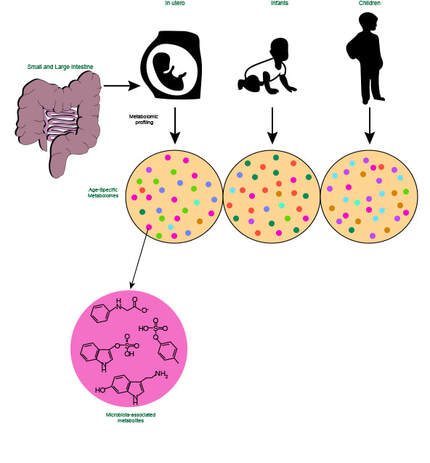
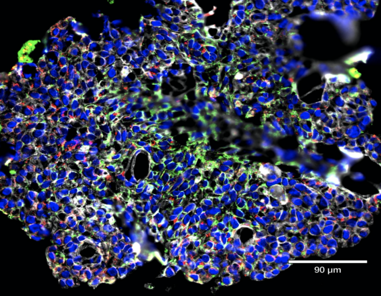
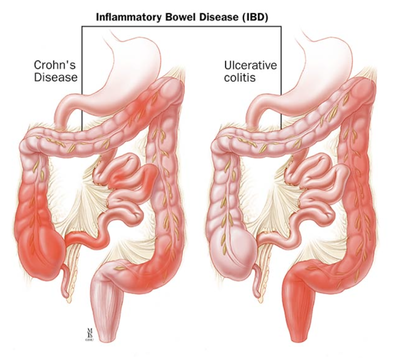
Intestinal immunity in health and diseaseOne of the main focuses of our group is to understand how mucosal homeostasis develops in infants and young children, particularly as it relates to development and maintenance of adaptive immunity. Using a combination of single cell techniques, we are studying intestinal immunity of fetal, premature and term infants and pediatric subjects.
Furthermore, we are interested in determining how mucosal homeostasis becomes dysregulated in intestinal diseases such as necrotizing enterocolitis and inflammatory bowel disease. |